New progress in morphology-dependent activity of silver nanostructures towards the electro-oxidation of formaldehyde was made by the research group headed by Prof. Lu Gongxuan of the LICP, CAS. The work is of great significance to the research on electrode materials in fuel cells.
The research group has systematically studied the catalytic property of the morphological controlled Pt (Electrochem. Commun, 2009, 11, 45-49), Rh(Chem. Commun. 2008, 6402-6404 ), Cu(Int. J. Hydrogen Energy, 2008, 33, 2225-2232), Au(Nanotechnology, 2008, 19, 275306) and Ag(Chem. Mater.,2008, 37, 514-515). In the present research, they prepared morphological controlled silver nanoproducts using the polyol process through varying the concentration of Na2S and investigated the relationship between the shape of Ag nanocatalyst and the activity for electro-oxidation of formaldehyde. The Ag nanoproducts were found to be efficient electrocatalysts for anodic oxidation of formaldehyde in alkaline solutions. Activities and selectivities were strongly dependent on the morphology of the silver nanoproducts. It was found that silver nanorods were more active than nanowires, nanopolyhedra and nanospheres.
Previous findings concerning the electrocatalytic activity of Ag indicate that relatively cheap Ag catalyst may be able to replace noble metal catalysts in fuel cells. The research has received financial supports from the National Basic Research Program of China and was published in Electrochemistry Communications (Electrochem. Commun., 2009, 11, 1255-1258).
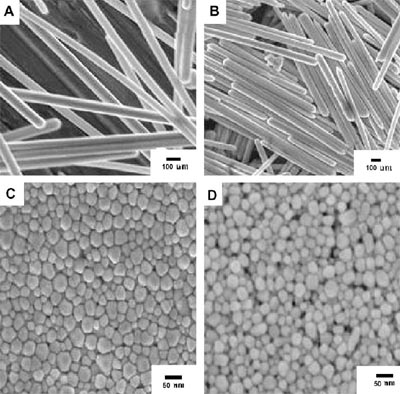
SEM images of silver nanostructures synthesized at different concentrations of Na2S: (A) silver nanowires, (B)silver nanorods, (C) sliver nanopolyhedra and (D) Ag nanospheres.
Abstract of the paper published inElectrochemistry Communications |

