|
Self-assembly of the [Fe(DABP)3]SO4 (DABP = 5,5'-diamino-2,2'-bipyridine) or [Fe(biPy)3]SO4 (bipy = 2,2'-bipyridine) complex with a tripodal tris(3-pyridylurea) ligand (L) results in a layered structure that includes a sulfate anion in the cleft of one L molecule. The two compounds, [Fe(DABP)3][SO4 L]·10H2O (2) and [Fe(bipy)3][SO4 L]·10H2O (2) and [Fe(bipy)3][SO4 L]·9H2O (3), show very similar sheets formed by the anionic units [SO4 L]·9H2O (3), show very similar sheets formed by the anionic units [SO4 L]2− and cationic building blocks ([Fe(DABP)3]2+ or [Fe(bipy)3]2+). However, there are different water clusters that link the adjacent layers in the two products, that is, water parallelograms and quasi “water cubes” in 2 versus single water molecules, water dimers, and hexamers in 3. The half-encapsulation of sulfate by a single L molecule contrasts with the previously reported full-encapsulation of the sulfate ion by two L molecules in [M(H2O)6][ SO4 L]2− and cationic building blocks ([Fe(DABP)3]2+ or [Fe(bipy)3]2+). However, there are different water clusters that link the adjacent layers in the two products, that is, water parallelograms and quasi “water cubes” in 2 versus single water molecules, water dimers, and hexamers in 3. The half-encapsulation of sulfate by a single L molecule contrasts with the previously reported full-encapsulation of the sulfate ion by two L molecules in [M(H2O)6][ SO4 L2] (1). This different anion encapsulation is traced to the hydrogen-acceptor properties of the pyridyl groups of L together with the hydrogen-bonding properties of the cation secondary coordination sphere for a solid-state packing optimization. In 1 the direct hydrogen bonding from the secondary coordination sphere of octahedral [M(H2O)6]2+ to L-pyridyl helps in the formation of an octahedral cation−anion coordination in the NaCl-type structure. In 2 and 3, crystal water instead of the cations has to satisfy the hydrogen-accepting demands of L. Consequently, a non-spherical and only partly water-surrounded half-encapsulated [SO4 L2] (1). This different anion encapsulation is traced to the hydrogen-acceptor properties of the pyridyl groups of L together with the hydrogen-bonding properties of the cation secondary coordination sphere for a solid-state packing optimization. In 1 the direct hydrogen bonding from the secondary coordination sphere of octahedral [M(H2O)6]2+ to L-pyridyl helps in the formation of an octahedral cation−anion coordination in the NaCl-type structure. In 2 and 3, crystal water instead of the cations has to satisfy the hydrogen-accepting demands of L. Consequently, a non-spherical and only partly water-surrounded half-encapsulated [SO4 L]2− anion allows for a closer approach of the [Fe(DABP)3]2+ or [Fe(bipy)3]2+ cations than the [SO4 L]2− anion allows for a closer approach of the [Fe(DABP)3]2+ or [Fe(bipy)3]2+ cations than the [SO4 L2]2− anion. Then, the similar cation and anion size in 2 and 3 with the Coulomb attraction confined to a two-dimensional plane leads to the formation of a hexagonal BN (or graphite) lattice. Competition experiments with different anions for compound 2 reveal that SO42− can be selectively crystallized against NO3−, OAc−, or ClO4−. L2]2− anion. Then, the similar cation and anion size in 2 and 3 with the Coulomb attraction confined to a two-dimensional plane leads to the formation of a hexagonal BN (or graphite) lattice. Competition experiments with different anions for compound 2 reveal that SO42− can be selectively crystallized against NO3−, OAc−, or ClO4−.
In summary, we obtained two supramolecular architectures, [Fe(DABP)3][SO4 L]·10H2O (2) and [Fe(bipy)3][SO4 L]·10H2O (2) and [Fe(bipy)3][SO4 L]·9H2O (3), via the self-assembly of [Fe(DABP)3]SO4 or [Fe(bipy)3]SO4 with a tris(3-pyridylurea) ligand (L). The two compounds show a similar layered structure, in which each ligand molecule includes a sulfate anion in its cleft via multiple hydrogen bonds. Alternative water parallelograms and quasi “water cubes” link the 2D layers formed by [SO4 L]·9H2O (3), via the self-assembly of [Fe(DABP)3]SO4 or [Fe(bipy)3]SO4 with a tris(3-pyridylurea) ligand (L). The two compounds show a similar layered structure, in which each ligand molecule includes a sulfate anion in its cleft via multiple hydrogen bonds. Alternative water parallelograms and quasi “water cubes” link the 2D layers formed by [SO4 L]2− anionic units and [Fe(DABP)3]2+ cations into a 3D network in compound 2, while in 3 single water molecules, water dimers and hexamers are found between the layers. FT-IR, PXRD, and elemental analysis reveal that SO42− can be selectively crystallized from an aqueous solution containing other oxoanions, such as NO3−, OAc−, and ClO4−, as competing anions. The encapsulation of sulfate by a single ligand molecule rather than two receptors is rationalized by the better solid-state packing mode of the complex cations [Fe(DABP)3]2+ and [Fe(bipy)3]2+ without sufficient secondary hydrogen-bond donors and half-encapsulated anions [SO4 L]2− anionic units and [Fe(DABP)3]2+ cations into a 3D network in compound 2, while in 3 single water molecules, water dimers and hexamers are found between the layers. FT-IR, PXRD, and elemental analysis reveal that SO42− can be selectively crystallized from an aqueous solution containing other oxoanions, such as NO3−, OAc−, and ClO4−, as competing anions. The encapsulation of sulfate by a single ligand molecule rather than two receptors is rationalized by the better solid-state packing mode of the complex cations [Fe(DABP)3]2+ and [Fe(bipy)3]2+ without sufficient secondary hydrogen-bond donors and half-encapsulated anions [SO4 L]2−. L]2−.
Inorg. Chem., 2009, 48 (21), pp 10249–10256
Corresponding author: Wu, BA, Chinese Acad Sci, Lanzhou Inst Chem Phys, State Key Lab Oxo Synth & Select Oxidat, Lanzhou 730000, Peoples R China
E-mail addresses: wubiao@lzb.ac.cn, janiak@uni-freiburg.de, yangxj@lzb.ac.cn
Click here to view the full text.
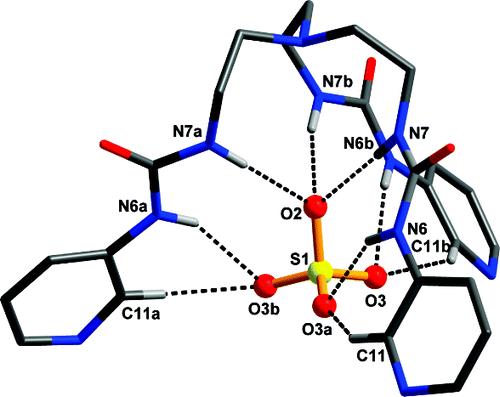
Sulfate ion inclusion in the [SO4⊂L]2- anionic unit in 2. Noninteracting hydrogen atoms are omitted for clarity. Symmetry code: a, -x+y, 1-x, z; b, 1-y, 1+x-y, z. |

