New progress in the design and construction of new asymmetric catalytic system was achieved by the research group headed by Prof. HUANG Hanmin, State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), CAS.
As an important research direction in the field of asymmetric catalysis, the design and construction of highly efficient asymmetric catalyst system has attracted significant interest.
Through taking advantage of the fact that the free proton source could promote the catalytic turnover for the Friedel-Crafts reaction, researchers have successfully established a cooperative catalytic system by the combination of an iron salt and a chiral phosphoric acid. In the cooperative catalytic system, there’s a Lewis acid site for activating the electrophile, a base site for activating the nucleophile through a hydrogen-bonding interaction, and a free proton source for accelerating the proton transfer. All these were incorporated into one chiral platform to achieve high asymmetric induction. The brand new concept for catalyst design has already been proven to be very effective in the asymmetric Friedel-Crafts alkylation of indoles with β-aryl-α´-hydroxy enones.
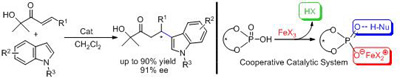
Catalytic system
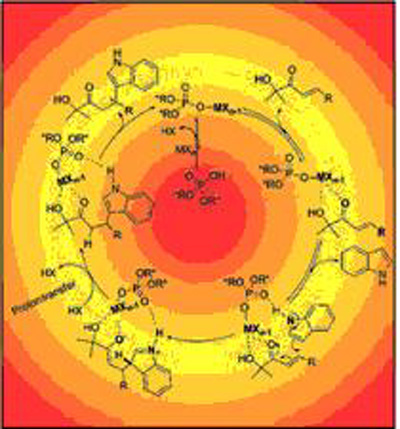
Catalytic reaction mechanism
The work has attracted wide attention in domestic and abroad since its publication in Chemistry-A European Journal (Chem. Eur. J. 2010,16(5), 1638). Moreover, the paper was published on the inside cover of the journal (Chem. Eur. J. 2010,16 (5)) and evaluated as one of the most accessed papers of the journal in December, 2009.(http://www3.interscience.wiley.com/cgi-bin/fulltext/123262633/PDFSTART)
Apart from the above-mentioned work, the research group has also achieved important progress in the synthesis of new chiral ligands. They have synthesized two kinds of new chiral diamines biisoindoline and hexahydrodibenzo[c,h][1,5] naphthyridine with rigid backbones. The effectiveness of biisoindoline as a chiral ligand was demonstrated by Ni (II)-catalyzed enantioselective Michael addition of malonates to conjugated nitroalkenes. The work was published in Organic Letters (Org. Lett., 2009, 11(20), 4536).
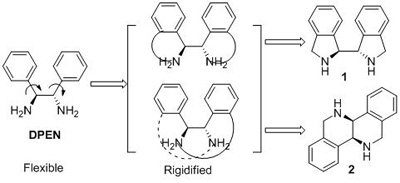
Design of ligands
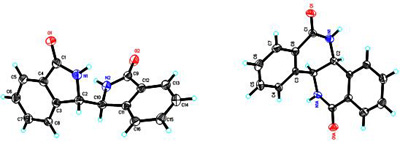
Crystal structure
Abstract of the paper published in Chemistry-A European Journal
Abstract of the paper published in Organic Letters
|

