Researchers of the State Key Laboratory of Solid Lubrication have developed a new and efficient way for the preparation of substituted benzo[b] furans. They have prepared a variety of substituted benzo[b]furans in good to excellent yields under the mild reaction conditions from o-(1-alkynylphenoxy)-1-phenylethanone under phase-transfer catalysis (PTC). This methodology accommodates simple experimental operations, inexpensive and environmentally benign catalysts, metal catalyst-free conditions, facile reagents and the possibility to conduct large-scale preparations. The development of carbon-carbon bond formation processes via an overall structural isomerization represents the most atom-economical approach.
The benzo[b]furan moiety has attracted widespread interest in view of its presence in natural products, and their biological and pharmacological activities. Much attention has been dedicated to the preparation of substituted benzo[b]furans.However, the direct carbocyclization of ortho-alkynyl phenyl ethers under PTC (phase-transfer catalyst) conditions for the synthesis of 2,3-disubstituted benzo[b]furan under metal-free conditions has not previously been reported.
The reaction has great application potential in industrial production due to its simple experimental operations, efficient and environmentally friendly catalysts and mild reaction conditions.
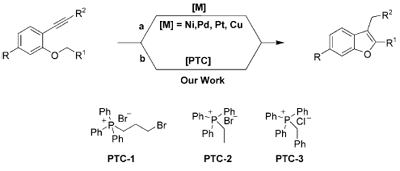
Transition metal-catalyzed or phase-transfer-catalyzed intramolecular cyclization of the corresponding ortho-alkynyl phenyl ether derivatives |

