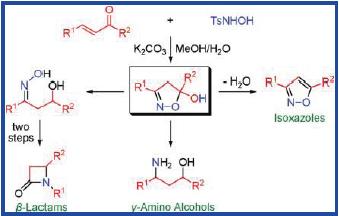
Researchers of the State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, CAS, have developed an efficient approach for the highly regioselective synthesis of a diverse array of 5-hydroxy-2-isoxazolines, in which TsNHOH was employed as an effective reactant for the conjugate addition to R,β-unsaturated carbonyl compounds and subsequent extrusion of sulfinate followed by the cyclization to afford the desired products.
This methodology tolerates a wide variety of functional groups, including 3-heterocycle, 3-styryl, and 3-ester enals/enones. Furthermore, 5-hydroxy-2-isoxazolines were demonstrated as versatile synthons for the syntheses of isoxazoles, β-hydroxy oximes, and N-aryl-β-lactams.
2-Isoxazolines are important heterocycles in organic and medicinal chemistry, which are frequently found in a diverse array of compounds, including biologically active natural products (e.g., cycloserin, acivicine) or drug candidates, chiral ligands, and intermediates in organic synthesis. Particularly, due to their ability to undergo facile reductive ringopening reactions, 2-isoxazolines are of interest as precursors for β-hydroxy ketones, β-hydroxy nitriles, β-amino acids, and γ-amino alcohols, etc. Therefore, numerous synthetic methods have been developed to produce 2-isoxazolines. Although these methods are widely used, some challenges still remain especially for regio- and stereoselectivities.
Abstract of the paper published in Journal of Organic Chemistry |

