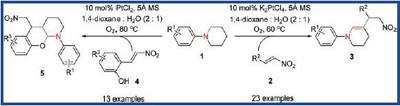
A mild platinum-catalyzed dehydrogenation of α, β-sp3 C-H bonds adjacent to the nitrogen of tertiary amines was successfully established by the researchers of Lanzhou University and the Lanzhou Institute of Chemical Physics (LICP), CAS.The in situ formed enamines initially serving as carbon-nucleophiles reacted with various nitroolefins, leading to the development of two one-pot protocols involving Michael addition-elimination and Michael addition-cyclization.
This platinum-catalyzed reaction provided an effective access to structurally divergent heterocyclic compounds. Additionally, the present reaction can tolerate a large number of substrates, including aromatic and aliphatic nitroolefins as Michael acceptors as well as various functionalized (E)-nitrovinylphenols as Michael acceptors/donors with significant structural variation.
This mild synthesis of enamine intermediates provides a pathway in one-pot manner to the synthetically useful trisubstituted enamines and chromano-[2,3-b]piperidines, which have received increased attention due to their medicinal potentials, especially as antiallergic and antiulcer drugs.
The full report of the results was published in J. Org. Chem. (J. Org. Chem. 2010, 75, 2893–2902).