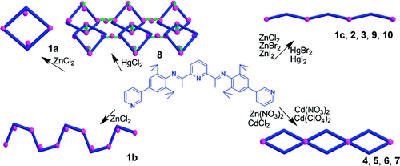
Researchers of Lanzhou University, Lanzhou Institute of Chemical Physics (LICP), CAS, and Northwest University recently reported 12 metal-organic coordination architectures of a ditopic diiminopyridine ligand 2,6-bis(1-(2,6-diisopropyl-4-(pyridin-3-yl)phenylimino)ethyl)-pyridine (L) with group 12 metal Zn(II), Cd(II), and Hg(II) ions. The compounds are structurally diverse, including five main structural motifs: 0D metallomacrocycle, 1D zigzag chain, 1D helical chain, 1D looped-chain, and 2D network. In the complexes, the ligand L shows four different conformation modes, which are responsible for the various structural topologies.
This study clearly demonstrates that the solvent system, the metal ion, and anion play important roles in determining the coordination modes of the metal centers, resulting in different building blocks in the assembly of the supramolecular structures. Photoluminescent studies indicate that the complexes exhibit enhanced and blue-shifted solid-state fluorescence at room temperature compared with ligand L.
Metal-organic frameworks (MOFs) from self-assembly of metal ions and multifunctional ligands have developed rapidly in recent years because of their fascinating structural topologies and potential applications as functional materials in the fields of catalysis, gas absorption, chirality, magnetism, nonlinear optics, and luminescence. In order to obtain MOFs with desirable structures and properties, many attempts have been made using different strategies.However, the key to successful design of intriguing MOFs should be the proper choice of metal center with preferred coordination geometries and intelligent ligand design
The research is helpful in understanding how the resulting coordination networks can be manipulated by varying the conformation of the ligand, coordination geometry of the metal, and structure of the anion and by utilizing various solvent systems, which is the key to successful design of intriguing MOFs.
The detailed report of the work is published in the recent issue of Crystal Growth & Design (Crystal Growth & Design, Vol. 10, No. 5, 2010).