A novel oxidative carbonylation reaction has been developed by researchers of Wuhan University and Lanzhou Institute of Chemical Physics, CAS. Under a balloon pressure of CO/air and at temperatures of 40→50℃, readily available arylboronic acid derivatives could be converted into their corresponding carbonylative products using [PdCl2(PPh3)2] as the catalyst precursor. It is the first example whereby arylboronic acid derivatives can be transformed into carbonylative products using air as the sole oxidant under mild conditions.
The transition-metal-catalyzed carbonylation involving CO gas is a fundamental chemical transformation. Theoretically, the Ar group could originate from an electrophile (ArX; Path I) or a nucleophile (ArM; Path II). However, the reaction in Path I usually requires relatively harsh conditions; for example, high temperature or high CO pressures.
Recently, oxidative carbonylation of ArM (M=H, In) with CO has emerged as a conceptually new alternative to “classic carbonylation processes”. The use of CO avoids the reluctant oxidative addition step and has the potential to accommodate milder reaction conditions. The oxidative carbonylation of ArH shows promise when catalyzed by transition metals, whereas the current transformation usually requires the use of directing groups. Furthermore, the oxidative carbonylation of arylindium derivatives requires the use of stoichiometric amounts of the oxidant desyl chloride. The current research is the first example of the oxidative carbonylation of arylboronic acid derivatives under balloon pressure of CO with air as the oxidant at 40→50℃.
Arylboronic acid derivatives are air and moisture stable, and are compatible with a broad range of common functional groups.However, there is no literature precedent of their oxidative carbonylation to form carboxylate esters. Furthermore, with respect to the cost and simplicity, air is the ideal oxidant for oxidative carbonylation. The oxidative carbonylation of amines or alcohols was investigated by employing air or oxygen as the oxidant. However, to the best of the researchers’ knowledge, no example has been reported regarding the oxidative carbonylation of arylmetal reagents by employing oxygen or air as the sole oxidant.
The work was published in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2010, 49, 3371 –3374).
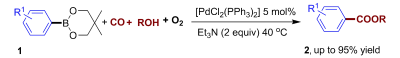
Abstract of the paper published in Angew. Chem. Int. Ed.

