In past decades, the synthesis of heterocycles has continued to attract the interest of synthetic chemists due to the number of these compounds that show antidepressant, antihypertensive, and hypoglycemic activities as well as other biological effects. Among these heterocycles, the five- and six-membered oxygenated or nitrogenated heterocycles are probably one of the most common structural subunits in numerous natural products. From simple to complex, these molecular frameworks are present in the structure of several biologically interesting compounds. In particular, dihydrofurans, dihydropyrroles, and dihydro-2H-pyrans are important intermediates for the synthesis of pharmaceuticals and biologically active molecules, such as lepadiformine, nicotine, phytane-type diterpenedilactones 3-7, citreoviral, (+)-anamarine, and (+)-ambruticin. Thus, a mild, metal-free, environmentally benign and atom economic protocol for the straightforward annulation of five-and six-membered heterocyclic rings is still of high demand.
Recently, Prof. LIANG Yongmin, a visiting professor of the Lanzhou Institute of Chemical Physics (LICP) of the CAS and co-workers have obtained highly substituted dihalogenated dihydrofurans, dihydropyrroles, and dihydro-2H-pyrans in moderate to excellent yields from simple starting materials by the electrophilic cyclization of 1,4-butyne-diols, 4-aminobut-2-yn-1-ols, and pent-2-yne-1,5-diols by electrophiles (I2, IBr, and ICl), during which several C-X (O, N, Cl, Br, I) bonds were formed.
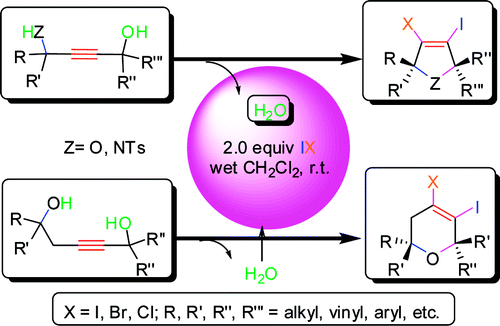
This method tolerates many alkyl, vinyl, aryl, and heteroaryl functional groups. Both halogen atoms generated from electrophiles were used effectively. The dihalogenated moiety can be readily introduced into the heterocycle in a position not easily obtained previously. Subsequent functionalization of the resulting heterocycles by palladium-catalyzed coupling reactions leads to a number of interesting five- and six-membered substituted skeletons. In addition, the presence of a trace amount of water is needed for this electrophilic cyclization.
The work was published in J. Org. Chem. (J. Org. Chem. 2010, 75, 5670–5678).
Abstract of the paper published in J. Org. Chem. |

