(DENG Youquan et al.)
New findings about the mechanism of low-temperature CO oxidation over supported Pt and Pd catalysts have been presented by the Center of Green Chemistry and Catalysis of the Lanzhou Institute of Chemical Physics (LICP) of the CAS.
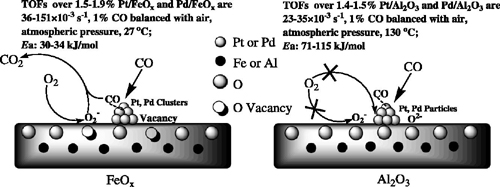
Researchers reveal the reason FeOx supported Pt and Pd catalysts possess higher activity for low-temperature CO oxidation. It is found that the partly reduced FeOx support is involved in the CO oxidation, acting as an oxygen supply.
The kinetic study of CO oxidation over supported Pt and Pd catalysts shows that Pt, Pd catalysts on different supports (FeOx and Al2O3) show differences in activation energy. FeOx-supported Pt, Pd catalysts showed almost the same Ea (activation energy), 30 and 34 kJ/mol, respectively. Nevertheless, Al2O3-supported Pt, Pd gave much higher activation energies, 115 and 71 kJ/mol, respectively. The striking difference in Ea suggested that the reaction pathways and/or rate-determining steps over those Al2O3-supported catalysts might be completely different when compared with FeOx-supported Pt and Pd. Those results partly illustrated the fact that FeOx-supported Pt and Pd catalysts exhibited superior CO oxidation activities when compared with Pt/Al2O3 and Pd/Al2O3.
H2–O2 titration and time-resolved CO titrations provide clear evidence that FeOx support is involved in the CO oxidation, acting as an oxygen supply. Thus, CO oxidation over Pt/FeOx, Pd/FeOx proceeds over two adjacent but different active sites (Pt, Pd for CO and FeOx for oxygen) with low activation energies (30–34 kJ/mol), which accounts for the dramatic difference in activity from Al2O3- and Fe2O3-supported Pt, Pd.
The research explains the reason that supported Pt/FeOx and Pd/FeOx, which are different from the traditional Al2O3 or SiO2 supported Pt and Pd catalysts, exhibited unusual activity for low-temperature CO oxidation.
Those findings and the exploration of the mechanism may open up new routes in the search for high activity in low-temperature CO oxidation over supported noble metal catalysts, in particular for Pt and Pd.
The work has received support from the National Natural Science Foundation of China (No. 20773146).
The detailed report of the work was published in the latest issue of Journal of Catalysis (Journal of Catalysis 274 (2010) 1–10).
Abstract of the paper published in Journal of Catalysis

