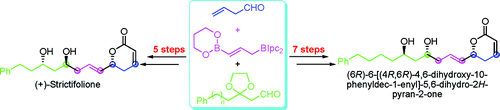
Researchers of the Lanzhou University and Lanzhou Institute of Chemical Physics (LICP) of the VAS have accomplished the total asymmetric syntheses of (þ)-strictifolione (1) and (6R)-6-[(4R,6R)-4,6-dihydroxy-10-phenyldec-1-enyl]-5,6-dihydro-2H-pyran-2-one (2). The synthesis proceeded in only five and seven steps with overall yields of 23 and 21%, respectively, from readily available 3-butenal.
The syntheses reported here represent the shortest asymmetric syntheses of 1 and 2 reported to date. Highlights of the synthetic venture included the successful utilization of organoboron reagents to introduce all the stereogenic centers in 1 and 2 and the RCM reaction to build up the δ-lactone.
Natural products possessing R-pyrone (R,β-unsaturated-δ-lactone) moieties often exhibit useful pharmacological properties. The broad range of biological activities reported for this class of compounds has been ascribed to their inherent tendency to act as good Michael acceptors. The relative and absolute stereochemistries of 1 were proposed based on spectroscopic analysis and revised by the same group after accomplishing its first total synthesis. (6R)-6-[(4R,6R)-4,6-Dihydroxy-10-phenyldec-1-enyl]- 5,6-dihydro-2H-pyran-2-one 2 is also one such natural product, isolated from Ravensara crassifolia by Hostetmann et al. along with a structurally similar compound (6S)-5,6-dihydro- 6-[(2R)-2-hydroxy-6-phenylhexyl]-2H-pyran-2-one 3, which were both shown to possess antifungal activity.
The work has received support from the Ministry of Science and Technology of China, National Natural Science Foundation of China and Program 111 of the Chinese Ministry of Education.
The findings have been published in J. Org. Chem. (J. Org. Chem. 2010, 75, 8234–8240).
J. Org. Chem. Paper

