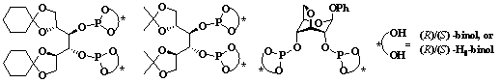
The synthesis of chiral phosphite ligands.(Image by WANG Lailai et al)
Synthesis of chiral functional molecules and materials through asymmetric catalysis is the frontiers in homogeneous catalysis. Design and synthesis of new chiral ligands is the key issue to be solved.
Researchers of the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), CAS, have worked on the problem for a long time and achieved certain progress. They have synthesized new chiral phosphate ligands derived from carbohydrate and successfully applied them to asymmetric catalytic reaction.
Researchers have stereoselectively synthesized new chiral aryl diphosphite ligands based on the pyranoside backbones of glucose and galactose. These ligands have been successfully applied to the Cu-catalyzed asymmetric conjugate addition of diethylzincto cyclic enones with up to 88% ee. (Adv. Synth. Catal. 2004, 346, 947–973)
Using the ligands, they have continued to research on asymmetric 1,4-conjugate addition reactions and asymmetric hydroformylation. They have stereoselectively constructed C-C bond and synthesized carbonyl-containing compounds, such as ketone or aldehyde with optical activity which are important drugs or organic synthesizing intermediates. (Tetrahedron: Asymmetry, 2005,16, 3198-3204, 2007, 18, 1899-1905).
Recently, based on the above work, with carbohydrate as starting materials, researchers have synthesized a novel series of diphosphite ligands. The ligands were successfully applied in the copper-catalyzed asymmetric conjugate addition of dialkylzincs to cyclic enones bearing different ring sizes with high enantioselectivities. It is found that the stereogenic centers of the D-mannitol skeleton and the axially chiral diaryl moieties of ligands had a synergic effect on the reactive activity and enantioselectivity of the reaction. (Tetrahedron: Asymmetry, 2010, 21, 2788-2793; 2010, 21, 2993-2998).
The work has provided a reference for the research on synthesis of chiral phosphate ligands and study of their structure-activity relationship. It has received support from the National Natural Science Foundation of China and Chinese Academy of Sciences.
Adv. Synth. Catal. Paper (Adv. Synth. Catal. 2004, 346, 947–973)
Tetrahedron: Asymmetry Paper (Tetrahedron: Asymmetry, 2005,16, 3198-3204)
Tetrahedron: Asymmetry Paper (Tetrahedron: Asymmetry, 2007, 18, 1899-1905)
Tetrahedron: Asymmetry Paper (Tetrahedron: Asymmetry, 2010, 21, 2993-2998)

