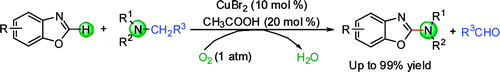
Schematic picture of the reaction.
Researchers of the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics (LICP), CAS, have developed an efficient and conceptually new method for oxidative amination of azoles with tertiary amines via copper-catalyzed C-H and C-N bond activation. This protocol can be performed in the absence of external base and only requires atmospheric oxygen as oxidant. The catalyst system is very simple and efficient, which opens a new way for using tertiary amines as nitrogen group sources for C-N bond formation reactions.
The transition-metal-catalyzed selective C-N bond formation reaction through C-H bond activation is an efficient method to facilitate the construction of complex amines. With respect to the nitrogen group sources, primary amines, secondary amines, chloroamines, and amides have been successfully installed into the corresponding substrates via C-H activation. Despite that remarkable success has been achieved, to the best of our knowledge, tertiary amines used as amino group sources for a direct amination reaction via C-H and C-N bond activation have never been reported. This limitation is probably due to difficulty in the cleavage of the C-N bond as compared to other C-X bonds.
In the current work, a new and efficient procedure for aerobic oxidative amination of azoles with tertiary amines as nitrogen sources has been put forward. It was catalyzed by a copper catalyst through the cleavage of two C-H bonds and one C-N bond under external-base-free conditions. The success of the present study not only provides a powerful method for the construction of new C-H amination reactions but also suggests a new strategy for C-N bond activation.
The work has received support from the National Natural Science Foundation of China and CAS. The detailed report was published in Org. Lett. (Org. Lett., Vol. 13, No. 3, 2011).
Org. Lett. Paper

