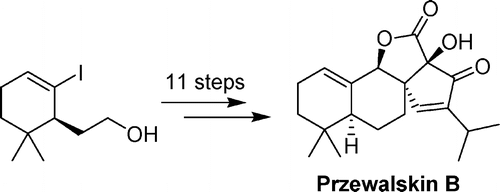
The first total synthesis of (-)-Przewalskin B has been achieved in 15 steps with 8.1% overall yield starting from the commercially available 4,4-dimethyl-2-cyclohexenone by researchers of Lanzhou University and Lanzhou Institute of Chemical Physics (LICP) of the CAS. The synthesis involves an INAS reaction, intramolecular aldol condensation, and lactonization as key steps to furnish the unique fused tetracyclic skeleton of the natural product.
Przewalskin B (1), a novel diterpenoid isolated from a Chinese medicinal plant SalVia przewalskii by Zhao et al. in 2007, was found to exhibit modest anti-HIV-1 activity with EC50 ) 30 μg/mL. The structure of 1 was revealed by means of comprehensive NMR spectroscopic analysis and a single-crystal X-ray study to have a fused tetracyclic skeleton containing a five-membered spiro ring and α-hydroxy-β-ketone lactone moieties. Due to its biological profile and intriguing structure, Przewalskin B (1) represents an attractive target for the synthetic community.
The work has received support from the Ministry of Science and Technology and National Natural Science Foundation of China. The detailed report has been published in Org. Lett. (Org. Lett., Vol. 13, No. 2, 2011).
Org. Lett. Paper

