Researchers of the Lanzhou Institute of Chemical Physics (LICP) and Dalian Institute of Chemical Physics (DICP) of the CAS have synthesized a series of novel N-heteroaromatic ring-containing R2NOH-type radical precursors. Through complexation with metal salts they were found to be able to remarkably improve the activity of the organic metal-free catalyst, and toluene could be efficiently converted with this kind of catalytic system.
This is the first report that a metal salt could improve the activity of metal-free organic molecule through complexation, which is contrary to the understanding of traditional organic metal complex catalysis. Further studies of the reaction mechanism and extensive application of this catalytic system for the other hydrocarbon oxidation are underway.
The catalytic system reported herein runs totally contrary to the traditional definition of an organic metal complex catalyst, in which a metal-free organic molecule acts as the catalytically active component while through complexation the metal ion acts as “ligand” to improve the catalytic ability of the organic molecule. Inert hydrocarbons such as toluene could be efficiently converted to, e.g., benzoic acid. Industrially, the oxidation of toluene to benzoic acid with O2 is a key step for synthesizing ε-caprolactamine in the Snia–Viscose process, and merely 15% of the toluene is converted with 90% selectivity to benzoic acid under the optimal conditions. Although many efforts have been made to improve the efficiency of toluene oxidation, a high efficiency could not be obtained under mild conditions without halogen ions, acidic solvents or powerful oxidant such as t-BuOOH
The work has received support from the National Natural Science Foundation of China. The detailed report has been published in Adv. Synth. Catal. (Adv. Synth. Catal. 2011, 353, 226 – 230).
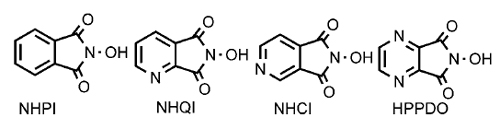
Structure of N-heteroaromatic ring-containing R2NOH derivatives
Adv. Synth. Catal. Paper

