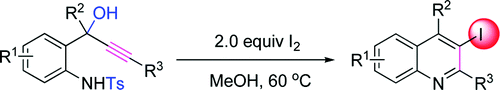Researchers at Lanzhou University and Lanzhou Institute of Chemical Physics have developed an efficient and highly regioselective protocol for preparing quinolines from readily available and simple starting materials.
The reaction is metal-free and can produce substituted 3-iodoquinolines in a single step in moderate to excellent yields. The resulting 3-iodoquinolines can be further functionalized by using known organopalladium chemistry, such as Sonogashira and Suzuki cross-couplings and the Heck reaction.
Quinoline derivatives are a major class of heterocycles which occur in various natural products. The quinoline skeleton is often used for the construction of many synthetic compounds with diverse pharmacological properties. In particular, halogen-containing quinolines are of special interest because the halogen atom sometimes plays an important role in the compound’s bioactivity. As a result, a number of attempts have been made to prepare and functionalize them since the late 1800s. The structural core of quinoline can be synthesized by various conventional methods. However, these classical syntheses often make use of elevated temperatures and prolonged reaction times and/or use of strong acids and bases, which cannot be applied to sensitive substrates. There are also many new metal catalyzed protocols for preparing quinolines. Thus, a metal free, mild, and environmentally benign protocol for preparing quinolines from readily available and simple substrates is still of high demand because of their extreme significance.
The work has received support from National Science Foundation and National Program on Key Basic Research Project of China (973 Program). The findings have been published in Org. Lett. (Org. Lett., Vol. 13, No. 10, 2011).
Org. Lett. Paper