The electronic states of Boron Difluoride (BF2) free radical was investigated in cooperation between Atomic Physics Group I at Institute of Modern Physics, Chinese Academy of Sciences (IMP) and the group of Professor Dennis J Clouthier at University of Kentucky, USA.
The near-ultraviolet band system of the jet-cooled BF2 free radical has been studied by a combination of laser-induced fluorescence (LIF) and single vibronic level wavelength resolved emission spectroscopies.
The radical was produced in a supersonic discharge jet using a precursor mixture of 1%–3% of BF3 or 10BF3in high pressure argon. A large number of bands were found in the 340–286 nm region and assigned as transitions from the 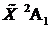 ground state to the lower Renner-Teller component of the
ground state to the lower Renner-Teller component of the 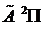 excited state, based on our previous ab initio potential energy surface predictions, matching the emission spectra Franck-Condon profiles of 11BF2and 10BF2, and comparison of observed and calculated boron isotope effects.
excited state, based on our previous ab initio potential energy surface predictions, matching the emission spectra Franck-Condon profiles of 11BF2and 10BF2, and comparison of observed and calculated boron isotope effects.
Several high resolution LIF bands have been rotationally analyzed providing ground state structural parameters of r0(BF) = 1.3102(9) Å and θ0)(FBF)= 119.7(6)°. The ground state totally symmetric vibrational energy levels of both boron isotopologues have also been measured and assigned up to energies of more than 8000 cm-1.
Although BF2 might be considered to be a “simple” free radical, understanding the details of its electronic spectrum remains a major challenge for both theory and experiment. It is indeed fortunate that the calculations predicted the pattern of K=0(∑) levels with such high fidelity that they allowed us to assign the very complex vibronic structure in the electronic spectrum of BF2 for the first time.
The results have been published in J. Chem. Phys. 135, 094305, 2011.
Weblink:http://jcp.aip.org/resource/1/jcpsa6/v135/i9/p094305_s1?isAuthorized=no
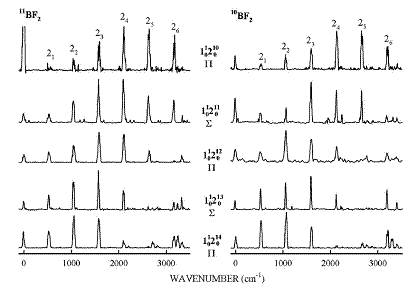
Fig. 1: The Franck-Condon pattern of the emission spectra of bands in the 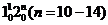 LIF progression. The transitions down to the bending overtone levels in the ground state show a regular intensity pattern, which is similar for both isotopologues (11BF2 on the left, 10BF2 on the right), aiding in the determination of the vibronic assignments of the LIF bands(Image by IMP).
LIF progression. The transitions down to the bending overtone levels in the ground state show a regular intensity pattern, which is similar for both isotopologues (11BF2 on the left, 10BF2 on the right), aiding in the determination of the vibronic assignments of the LIF bands(Image by IMP).
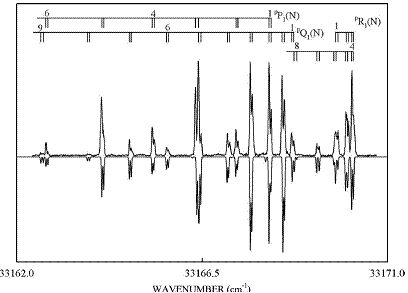
Fig. 2: The high resolution LIF spectrum of the band 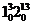 level of 11BF2(top) with assignments. Each line in the spectrum is split into two components, due to the presence of the unpaired electron. The bottom spectrum is a simulation of the observed spectrum, using the constants obtained from the rotational analysis (Image by IMP).
level of 11BF2(top) with assignments. Each line in the spectrum is split into two components, due to the presence of the unpaired electron. The bottom spectrum is a simulation of the observed spectrum, using the constants obtained from the rotational analysis (Image by IMP).

