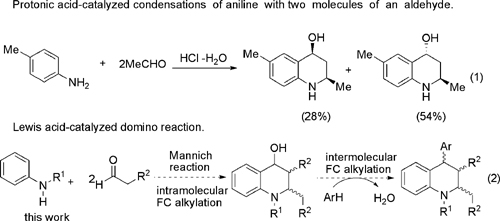
Proposed domino Mannich/intramoleculer FC alkylation/intermolecular FC alkylation reactions.
A simple method for a one-pot domino Mannich/intramolecular Friedel–Crafts alkylation/intermolecular Friedel–Crafts alkylation reactions by using iron(III) chloride as catalyst have been developed by researchers from Lanzhou University and Lanzhou Institute of Chemical Physics of the CAS, which leads to the synthesis of 1,2,3,4-tetrahydroquinoline derivatives.
The advantages of this method include good substrate generality, mild conditions, environment friendly catalyst and easy availability of starting materials. This strategy provides a pathway in one-pot manner to the synthetically useful tetrahydroquinolines.
Tetrahydroquinoline derivatives are an important class of biologically active compounds, which are widely used in organic synthesis and pharmaceutical chemistry. Currently, much effort in this area is focused on constructing polysubstituted tetrahydroquinolines. However, to the best of the researchers’ knowledge, intermolecular Friedel–Crafts (FC) alkylation to construct polysubstituted tetrahydroquinolines has been rarely reported. Domino reactions are attractive to industrial and laboratory chemists because of their potential to save solvents, reagents, time and energy. The researchers are persistently interested in domino reactions to synthesize various functionalized heterocyclic compounds.
The work has received support from the National Science Foundation and the Fundamental Research Funds for the Central Universities. The findings have been published in Org. Biomol. Chem. (Org. Biomol. Chem., 2011, 9, 5028–5033).
Org. Biomol. Chem. Paper

