Indoles are often used for the construction of many biologically active compounds with diverse pharmacological properties. The structural core of indoles can be synthesized by various classical methods. However, the functional group tolerance and the starting material availability often limit these syntheses. In some cases, it remains difficult to prepare suitably substituted indoles by standard indole-forming reactions. Thus, more benign protocols for preparing indoles from readily available and simple substrates still need to be actively perused.
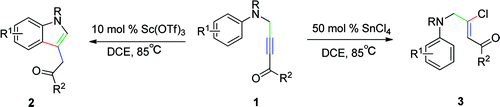
Researchers at Lanzhou University and Lanzhou Institute of Chemical Physics of the Chinese Academy of Sciences have realized a Sc(OTf)3-catalyzed intramolecular Friedel Crafts reaction for the synthesis of highly substituted indole derivatives from 5-(arylamino)pent-3-yn-2-ones. In addition, a SnCl4-mediated protocol for highly Zselective CCl bond formation was also successfully developed. The resulting CCl bond can be further exploited using cross CN coupling reactions.
The work has received support from the NSF, National Program on Key Basic Research Project of China (973 Program) and 111 Project.
The findings have been published in J. Org. Chem. (J. Org. Chem. 2011, 76, 8329–8335).
J. Org. Chem. Paper