Hydrogen is an ideal energy carrier. Photocatalytic hydrogen production (PHP) using a heterogeneous photocatalyst has been studied extensively as a potential method to supply hydrogen from sunlight and water. Though water is abundant on the earth, pure water is scarce because 93% of the earth’s water is in oceans and inland seas. Although PHP from seawater would be important to practical application, few studies on PHP from seawater have been made.
Researchers from Nanchang University and Lanzhou Institute of Chemical Physics of the Chinese Academy of Sciences (CAS) have investigated PHP over ZnS1-x-0.5yOx(OH)y-ZnO using sulfide ion (Na2SeNa2SO3) as an electron donor from NaCl saltwater, since NaCl is a major component of natural seawater and sulfide ion is a common pollutant.
It is found that NaCl can affect markedly the activity for PHP, depending on NaCl concentration. When NaCl concentration is lower, the activity is lower than that in pure water, whereas when NaCl concentration is higher, the activity is higher than that in pure water. The activity at 3.0 mol L-1 NaCl is 1.72 times as high as that at 0 mol L-1 NaCl. This suggests that with the photocatalyst and electron donor, a high-efficient reaction system of saltwater can be constructed, which is very important to practical application. NaCl decreases not only the surface charge of ZnS1-x-0.5yOx(OH)y-ZnO but also the surface hydration. These two factors can markedly influence the activity of ZnS1-x -0.5yOx(OH)y-ZnO. The impregnated photocatalyst (with Na2SeNa2SO3) exhibits much higher activity than non-impregnated one. This can be attributed the fact that ZnO is partly transformed into ZnS.
The work has received support from of the National Basic Research Program of China, the National Nature Science Foundation of China, Specialized Research Fund for the Doctoral Program of Higher Education of China, the Natural Science Foundation of the Jiangxi Province and Research Fund of Education Ministry of Jiangxi, China.
The findings have been published in International Journal of Hydrogen Energy (International Journal of Hydrogen Energy 36 (2011) 10565-10573).
International Journal of Hydrogen Energy Paper
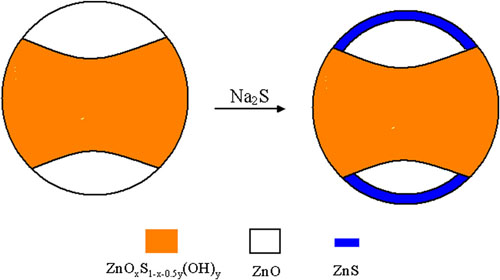
Schematic illustration of change of composition and structure of ZnOxS 1-x -0.5y(OH)y-ZnO after impregnating with 0.10 mol L-1 Na2S and 0.040 mol L-1 Na2SO3

