Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have synthesized a series of acidic-functionalized ionic liquids and applied them to the hetero-Michael addition of nitrogen, sulfur and oxygen nucleophiles to α,β-unsaturated ketones under under metal-free and solvent free conditions.
Notably, 1-methylimidazolium p-toluenesulfonic was found to be the most efficient catalyst. Additionally, the catalytic system successfully realized “homogeneous catalysis, two-phase separation” and has wide substrate scope including aromatic and aliphatic nucleophiles. Moreover, good to excellent yields (up to 99%) could be obtained for the three kinds of hetero- Michael addition reactions at room temperature. This process thus represents a greener pathway for the hetero-Michael reactions.
Michael and hetero-Michael addition are powerful reactions for the formation of carbon–carbon and carbon–hetero atom bonds. Moreover, the addition products such as b-amino, bthio and b-oxy ketone functionalities are important synthetic intermediates. Normally, either the donor or the acceptor component needs to be activated in hetero-Michael addition reactions. The classical method to achieve this has been deprotonation of the nucleophile with strong bases. And important advances have been made with Lewis acid catalysts, which activate the acceptor components and allow hetero-Michael addition to proceed under much milder conditions. In this respect, numerous homogeneous and heterogeneous catalysts have currently been developed. Both homogeneous catalysis and heterogeneous catalysis have their own advantages and disadvantages. To preserve the benefits of a homogeneous catalyst while co-opting the primary benefits of a heterogeneous catalyst, one strategy is to use functionalized ionic liquids as the catalyst, whereby the system could realize “homogeneous catalysis, two-phase separation”.
Although several kinds of ionic liquids have been used for the transformation,20 most are non-functionalized ILs and the ILs were usually used as solvents or their usage amount was too large for the catalytic system. Moreover, rarely reported catalysts were applied to investigate three different types of nucleophiles in detail. Therefore, a milder, environmentally benign catalyst system that can tolerate more nucleophile classes for functionalized Michael acceptors still remains to be explored.
The work has received support from the National Key Basic Research Project of China. The findings have been published in Org. Biomol. Chem.(Org. Biomol. Chem., 2012, 10, 346–354).
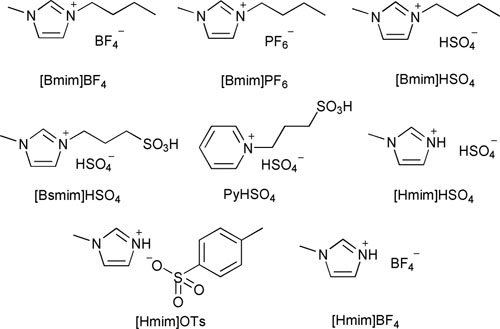
Acidic-functionalized ionic liquids used in the hetero-Michael reaction.
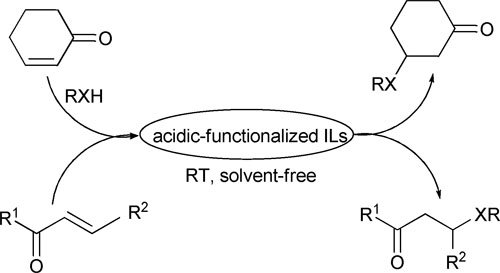
Hetero-Michael reactions of nitrogen, sulfur, oxygen nucleophiles with α,β-unsaturated enones

