As a new derivative of graphene, fluorinated graphene possesses both high strength of graphene and new interfacial and physical chemical properties, including reduced surface energy, stable chemical property, improved hydrophobic property and band-gap opening due to the introduction of fluorine atoms. Moreover, fluorinated graphene is resistant to high temperature and shows properties similar to those of poly(tetrafluoroethylene). And it is called “two-dimensional teflon”. These special properties of fluorinated graphene make it promising in many fields, such as interface, nano-electronic devices, lubricating materials and so on.
At present, fluorinated graphene is usually synthesized through the fluorination of graphene obtained by micro-mechanical cleavage or chemical vapor deposition using XeF2 as the fluorinating agent. However, the use of expensive and highly toxic fluorinating agent limits the practical application of the method.
The research group headed by Professor WANG Jinqing at the State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), has successfully prepared high quality fluorinated graphene using commercially available graphite fluoride as raw materials and cheap N-methyl pyrrolidone as the exfoliating reagent via simple thermal and ultrasonic treatments. It is found that the time of ultrasonic process can tune the content of fluorine in final product, and some physical and chemical properties of fluorinated graphene are systematically investigated. The work has been published in Journal of Materials Chemistry(2012, 22, 16950-16956).
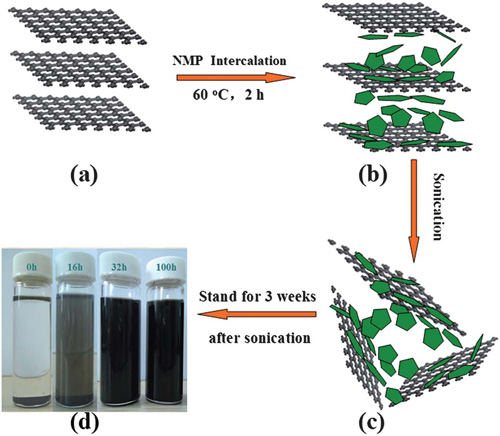
Schematic illustration of the NMP intercalation and exfoliation processes for preparing fluorinated graphene.(Image by WANG Jinqing et al.)
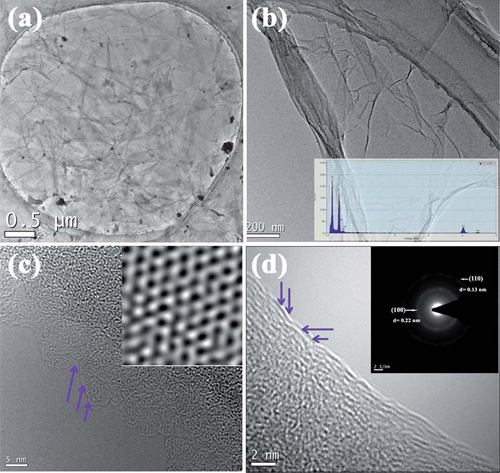
In addition, adopting graphene oxide and hydrogen fluoride as raw materials, the group has also prepared high quality fluorinated graphene of which the fluorination degree can be tuned via hydrothermal reaction. Researchers have also investigated the electric conductivity and band-gap opening of the fluorinated graphene and proposed a possible fluorinated mechanism based on the Raman spectra analysis. The work has been published online in Carbon(DOI: 10.1016/j.carbon.2012.07.026).
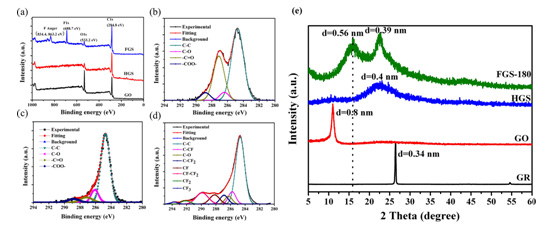
(a) Survey XPS spectra of the as-prepared GO, HGS, and FGS samples. (b-d) Gaussian fitting results of the C1s core lines of GO, HGS, and FGS. (e) Typical XRD patterns of GR, GO, HGS and FGS samples. (Image by WANG Jinqing et al.)

