Polycyclic aromatic hydrocarbons (PAHs) have been of great interest especially in materials science because of their utility in organic electronics, such as light-emitting diodes, field-effect transistors, and solar cells. In particular, fluorine derivatives are notable structural motifs for the diverse PAH derivatives having various applications as dyes or optical brightening agents. A great number of methods have been developed for the synthesis of fluorenes. Although these methods are effective for the synthesis of fluorenes, they have certain drawbacks, for example, a strong acid medium or a stoichiometric amount of Lewis acid sometimes is required. For these reasons, a direct and preparative method for functionalized PAHs is considered to be highly urgent.
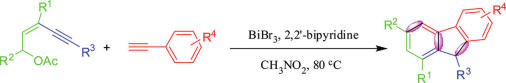
Researchers from Lanzhou University and Lanzhou Institute of Chemical Physics have developed a straightforward method for the synthesis of multisubstituted fluorene with (Z)-pent-2-en-4-yl acetates and ethynylarenes catalyzed by BiBr3 via domino reaction, which involved intermolecular electrophilic addition and cycloisomerization aromatization. Variation of substituted groups was proven possible and this reaction can proceed smoothly in moderate to good yields.
The work has received support from the National Basic Research Program of China (973 Program), National Basic Research Program of China (973 Program) and “111” Project. The findings have been published in J. Org. Chem. (J. Org. Chem. 2012, 77, 2064−2068).