There is much interest in the design and synthesis of well-defined concave space to encapsulate guest species (anions, cations or neutral molecules) due to the great potential of such systems in biological, analytical and catalytic applications. The artificial organic columnar structures are among the most studied molecular capsules, and many covalent cylindrical molecules have been employed in this respect. However, the synthesis and separation of such host molecules is much challenging. Moreover, the flexibility of hosts is very important in biological systems, in which recognition of a substrate may result in a conformational change that is of major significance for the function to be realized.
Researchers from Northwest University and Lanzhou Institute of Chemical Physics have been working on anion coordination and investigated the self-assembly of a bis–trisurea ligand (L) with six tetraalkylammonium salts. Remarkably, the ligand exhibits considerable flexibility to form ‘barrel’ structures that encapsulate multiple R4N+ guests while binding the anions at the outside. The size of both the R4N+ cation and the halide anion (Clor Br) has great influence on the final structure.
This work further proves the ability of oligo-urea ligands to direct the formation of rich anion-based supramolecular architectures.
The work has been published in Chem. Commun. (Chem. Commun., 2012, 48, 3097–3099).
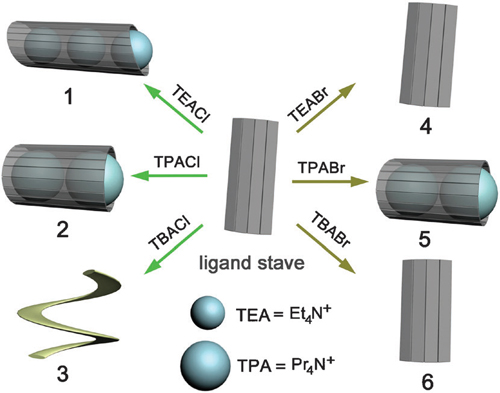
Summary of the ion-pair induced self-assembly of complexes 1–6. The ligand ‘stave’ underwent dramatic conformational changes when treated with different tetraalkylammonium halides to form the ‘barrel’ (complexes 1, 2 and 5), ‘stave’ (4 and 6), and foldamer (3) structures.

