Methanol electro-oxidation, as a promising solution to future energy problems, has received considerable attention due to the possible applications in direct methanol fuel cells (DMFCs). Over the past decades, platinum is considered as the most suitable catalyst for methanol oxidation reaction, but a major problem is the poisoning of platinum by CO-like species generated during the electro-catalytic process. Therefore, how to keep its activity and durability is of paramount importance.
Researchers at Lanzhou University and Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have prepared Au-Pt alloyed particles with cauliflower-like microstructures on ITO substrates via an electrochemical approach. In comparison, the film of pure Au exhibits dendrite-like structures and the SEM images of pure Pt appear to be porous nanoflower-like structures under the same electrodeposition conditions. The Au-Pt alloyed particles with different Pt/Au ratios can be modulated by controlling the molar ratios between metal precursors in the electrolyte. CV analysis demonstrates that the formation of the Au-Pt alloyed microstructures is attributed to the occurrence of gold cores due to fast reduction and subsequent simultaneous deposition of Au and Pt atoms around the cores. The electrocatalytic activities for methanol oxidation of the resulting Au-Pt alloyed electrodes were investigated in H2SO4 media. From the measurements of CV and chronoamperometry, the electrocatalytic efficiency is in the order of Pt4Au1 > Pt > Pt1Au1. The Au atoms in Au-Pt alloys promote the activity of methanol electro-oxidation mainly through electronic effect. In addition, Pt4Au1 has enhanced kinetics of methanol electro-oxidation than monometallic Pt.
Because the prepared Au-Pt alloy particles have unique structural and catalytic properties, they will find potential applications in the fabrication of novel micro/nanostructures and DMFCs.
The work has received support from the Natural Science Foundation of Gansu Province, China, and the Fundamental Research Funds for the Central Universities.
The findings have been published in International Journal of Hydrogen Energy(International Journal of Hydrogen Energy37 (2012) 4088-4097).
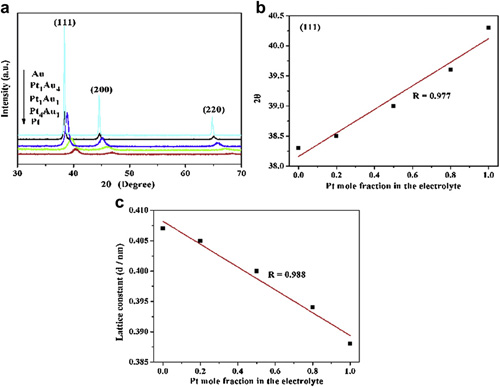
XRD patterns (a) (111) diffraction peak positions (b) and lattice constant (c) of the samples

