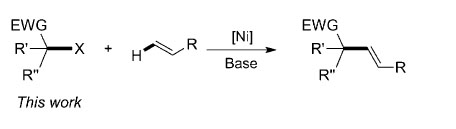
Since its development in the early 1970s, the palladium-catalyzed Heck reaction has become a powerful tool for the alkenylation of aryl and alkenyl electrophiles in chemical synthesis [Eq. (1)]. Thus far, alkyl halides have had limited application in Heck-type reactions, with only isolated examples available for primary alkyl halides and both secondary and tertiary alkyl halides remaining almost unstudied. This may be because of facile β-hydride elimination on palladium catalysts with such species.
Researchers at Wuhan University and Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have demonstrated the first nickel-catalyzed Heck-type reaction of secondary and tertiary acarbonyl alkyl bromides with olefins under mild conditions. Several substituted styrenes and various 1,1-diaryl alkenes were found to be suitable substrates for this a-alkenylation.
This work opens up a new approach for the construction of aalkenyl carbonyl compounds. Tentative mechanistic studies suggest that this reaction is likely to proceed by a single electron transfer mechanism, with a catalytic cycle involving NiI and NiII complexes as the catalyst species.
The findings have been published in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2012, 51, 3638 –3641).

