As a kind of typical interface materials of soft matter, polymer brushes have been widely used in tuning surface wettability and adhesion, lubrication, biocompatibility, anti-protein adhesion, anti-biofouling and so on. (Chem. Soc. Rev., 2011, 40, 4244-4258) It is of great theoretical and practical significance to prepare polymer brushes with tunable thickness, composition and structure via controllable surface initiated radical polymerization.
Surface initiated atom-transfer radical polymerization (ATRP) is one of the major approaches used to assemble polymer brushes. However, there exist several disadvantages concerning the method, such as the poor tunability of the growth of polymer brushes, use of inert gas, use of large amount of monomer solution, etc.
The surface/interface research group at the State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, has been long engaged in the study of assembly of polymer brushes.
They have, for the first time, accomplished surface initiated electrochemically mediated ATRP (SI-eATRP) on the conducting substrate, Au. (Angew. Chem. Int. Ed., 2012, 51, 5092-5095). With their new approach, the use of inert gas and waste of monomer can be avoided. The reaction can be carried out just in air and the monomer solu tion can be reused for several times. Moreover, they have achieved the in-situ-tracking of the process of surface initiated ATRP. (Macromol. Rapid Commun.2013, 34, 246-250) But this method is not quite applicable to non-conducting substrates and the tunability of the polymerization is not satisfactory.
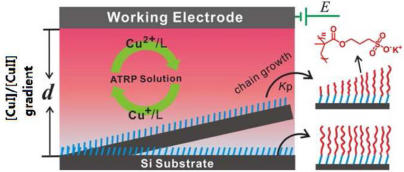 |
| Schematic Illustration of Using Diffusion to Control eATRP for Surface Modification (Image by ZHOU Feng et al.) |
Recently they have realized SI-eATRP on non-conducting substrates through the diffusion of catalysts in limited space.
They have successfully exploited concentration gradients originating from CuI diffusion from an electrode surface to initiate ATRP on nonconducting substrates. An adequate concentration of deactivator CuII in the polymerization can be rationally adjusted by the applied potentials or the gap d between the two electrodes to ensure a fast rate of deactivation, thereby suppressing unwanted termination reactions for many hours and brush growth with significantly enhanced control. The findings have been published in J. Am. Chem. Soc.(2013, 135, 1708–1710).
The approach can not only give more insights into surface initiated ATRP kinetics but also provide the easiest way for forming surface gradients by simply changing the sample conformation.

