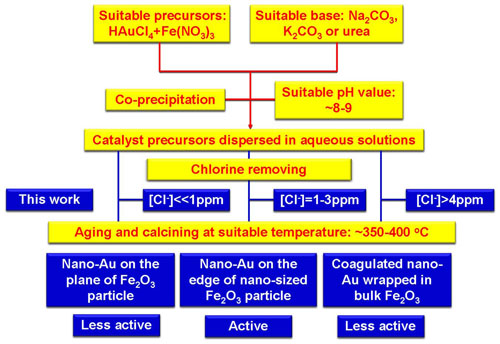
An illustration for the chlorine concentration mediated preparation of active Au/Fe2O3 catalyst with co-precipitated method. Yellow: Known parameters for active nano-Au catalyst preparation; Blue: New parameters revealed in this work.(Image by SHI Feng et al.)
Nano-gold catalysis has received fantastic attention since the discovery of the prominent behavior of nano-gold in CO low-temperature oxidation. However, the controllable preparation of nano-gold catalyst remains a challenge. Researchers at R&D Center for Green Chemistry and Catalysis, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have showed that controllable preparation of active nano-gold catalyst can be achieved using chlorine as an indicator.
By tracing the chlorine concentration in the washing step, a series of Au/Fe2O3 catalysts were prepared with co-precipitation method. The applying of these catalysts in CO oxidation and reductive nitrobenzene N-alkylation suggested the active catalysts were prepared from solutions containing ~2 ppm chlorine. The catalytic activity dropped dramatically if the chlorine concentration is >4–6 or <<1 ppm. Extensive characterizations revealed that the active catalyst was composed by nano-gold on the edge of Fe2O3 particle with 8.92 Au-Au coordination numbers. Although the real role of chlorine in the variation of catalyst structure and activity was still ambiguous, the current results should promote the controllable preparation of active nano-Au catalyst.This finding has been published in Sci. Rep., (2013, 3, 1503).

