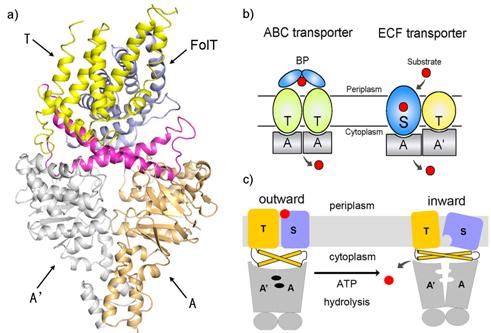
Energy-coupling factor (ECF) transporters belong to a new family of ATP-binding cassette (ABC) transporters. The different organizational and functional properties of ECF transporters imply a unique transport mechanism which remains largely unknown. Recently, researchers from Chinese Academy of Sciences reported the structure of the folate ECF transporter complex-the first structure both for ECF transporters and folate transporters and disclosed a novel transport mechanism (Fig. a and c).
ATP-binding cassette (ABC) transporters, composed of importers and exporters, form one of the biggest protein superfamilies that transport a variety of substrates across the membrane harnessed by ATP hydrolysis. Most ABC transporters are composed of two transmembrane domains (TMD) and two cytoplasmic nucleotide-binding domains (NBD). As a new member of ABC superfamily, each ECF transporter is constituted by an energy-coupling module consisting of a transmembrane T protein (EcfT) and two nucleotide-binding proteins EcfA and EcfA’, and another transmembrane substrate-specific binding protein S (Fig. b). The structures of several S proteins were reported previously, revealing the molecular basis for substrate specific recognition. However, the transport mechanism of ECF transporters is unknown because of the lack of complex structure information.
Researchers led by Dr. ZHANG Peng from the Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences reported the first structure of the ECF family transporter complex-a 3.0 Å resolution folate ECF transporter. This structure was captured in an inward-facing, nucleotide-free conformation with no substrate bound. The overall structure of EcfT adopts an “L” shape. The folate-binding protein FolT is nearly parallel to the membrane and is bound almost entirely by EcfT which connects to EcfA and EcfA’ through two coupling helices. Two conserved XRX motifs from the coupling helices of EcfT play a vital role in energy coupling by docking into EcfA-EcfA’. A transport model including a substantial conformational change of FolT was proposed based on biochemical and biophysical analysis. Determination of this structure takes the crucial step toward dissecting the molecular mechanism underlying the ECF transporters and will shed new light on understanding the process of vitamin uptake and antibiotics design.
This work entitled “Crystal structure of a folate energy-coupling factor transporter from Lactobacillus brevis” has been published online in NATURE on April 14. (Another ECF transporter complex structure was reported by SHI Yigong Group from Tsinghua University in the same issue and they drew a similar conclusion.)
The research was funded by the Ministry of Science&Technology of China, Chinese Academy of Sciences and Science&Technology Commission of Shanghai Municipality. Data were collected at BL17U of the Shanghai Synchrotron Radiation Facility (SSRF).
CONTACT:
ZHANG Peng
National Key Laboratory of Plant Molecular Genetics
Institute of Plant Physiology and Ecology,Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Tel: 86-21-54924219; Email:
pengzhang01@sibs.ac.cn 
