Methylornithine synthase (PylB) belongs to the family of radical SAM enzymes which converts (2S)-lysine to (2R,3R)-3-methylornithine in a radical mechanism. In this paper, the mechanism of lysine mutase reaction catalyzed by PylB has been studied by using quantum mechanics/molecular mechanics approach. The calculations reveal that the PylB-catalyzed reaction follows a fragmentation–recombination mechanism involving seven elementary reaction steps. Both the hemolytic cleavage of Cα–Cβ bond of lysine and the ligation of glycyl radical with aminobutene are possible rate limiting, corresponding to the calculated energy barriers of 23.0 and 24.1 kcal/mol, respectively. The intramolecular rotation of a fragment (aminobutene) can well explain the stereochemistry of the final product. Asp 279 functions as a general acid/base, and the other pocket residues such as Asp112, Arg235, and Ser277 form a network of hydrogen bonds responsible for orientation of the substrate. Additional Information:Author Information: Wenyou Zhu, Yongjun Liu, Rui ZhangCorrespondence: yongjunliu_1@sdu.edu.cn. 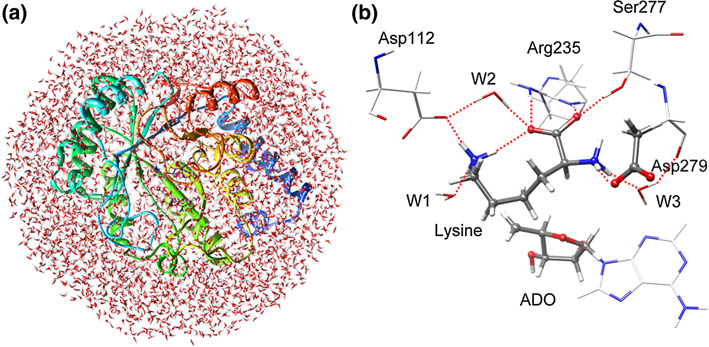
Fig. Optimized structures of solvated model (a) and active site (b) | |

