Aspartate ammonia lyase (Asp) is one of three types of ammonia lyases specific for aspartate or itsderivatives as substrates, which catalyzes the reversible reaction of l-aspartate to yield fumarate andammonia. LIU Yongjun and his team studied the catalytic mechanism of Asp by using combined quantum-mechanical/molecular-mechanical (QM/MM) approach. The calculation results indicate that the overallreaction only contains two elementary steps. The first step is the abstraction of Cβproton of L-aspartateby Ser318, which is calculated to be rate limiting. The second step is the cleavage of Cα-N bond of L-aspartate to form fumarate and ammonia. Ser318 functions as the catalytic base, whereas His188 is adispensable residue, but its protonation state can influence the active site structure and the existingform of leaving amino group, thereby influences the activity of the enzyme, which can well explain thepH dependence of enzymatic activity. Mutation of His188 to Ala only changes the active site structureand slightly elongates the distance of Cβproton of substrate with Ser318, causing the enzyme to remainsignificant but reduced activity. 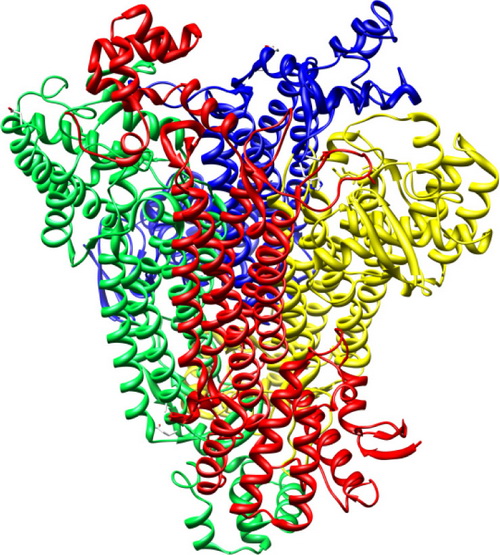
Figure.Overall structure of AspB. Four subunits are colored yellow, blue, red, andgreen, respectively. Additional Information: 1 Author Information: Jing Zhang, Yongjun Liu. Correspondence: yongjunliu 1@sdu.edu.cn 2 Published: Journal of Molecular Graphics and Modelling 51 (2014) 113–119 |

