Amides are not only recognized as fundamental structural motifs in myriad of natural products, pharmaceuticals, functional materials, and agrochemicals, but also serve as attractive precursors in a variety of organic transformations. Transitional metal catalyzed hydroaminocarbonylation of alkenes is an important method to synthesize amides and 100% atom economy can be achieved. However, compared with the study on hydroformylation, the report on transitional metal catalyzed hydroaminocarbonylation of alkenes is rare. This is mainly because that the effective catalytic reactor in this catalytic system is triggered by [M-H]. [M-H] can only be formed under relatively acid condition and aliphatic amines which are more basic (pKb<5) may inhibit the generation of [M-H], thus preventing the N-alkylsubstituted amides being formed.
Thus, the development of solutions to overcome the inherent side effect incurred by the strong basicity of aliphatic amines would be highly desirable to increase the range of amides which may be accessed.
The research group headed by Prof. HUANG Hanmin at the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences (CAS) has developed a new and efficient protocol for implementation of the palladium-catalyzed hydroaminocarbonylation of simple alkenes with a variety of aminals, which successfully overcomes the difficulty of using aliphatic amines and allows synthesis of a broad range of N-alkyl-substituted amides under mild reaction conditions.
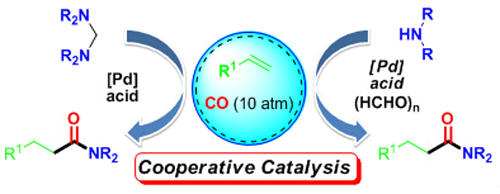
Cooperative catalysis developed by HUANG et al.
On the basis of this method, a cooperative catalytic system through the synergistic combination of palladium, paraformaldehyde, and acid was also established for promoting the hydroaminocarbonylation of alkenes with both aliphatic and aromatic amines.
This study not only provides an efficient method to realize the hydroaminocarbonylation with both aliphatic and aromatic amines under mild reaction conditions, but also paves the way for establishing new C-N bondformation reactions by using this cooperative catalysis. Studies aimed at gaining a detailed mechanistic understanding of this reaction and the application of this strategy in other reactions are currently in progress.
The work has received support from the National Natural Science Foundation of China and the “135” Planning of LICP.
The findings have been published in Angew. Chem. Int. Ed.2015, 54, 7657 –7661.
Key words: alkenes;amines;aminals;palladium;synthetic methods
Contact: HUANG Hanmin
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences
Lanzhou, 730000 (China)
E-mail: hmhuang@licp.cas.cn

