Abstract: A new strategy for selective insertion of metal carbenes into C-N bond has been developed via Pd-catalyzed C-N bond activation. A series of aminals and alpha-diazoesters with different substituents were successfully incorporated even in 0.1 mol % of catalyst under mild conditions, affording a wide range of alpha,beta-diamino acid esters with quarternary carbon-centers. Preliminary mechanistic studies uncovered that the unique electrophilic cyclopalladated species could easily react with diazoacetates to generate a Pd-carbenoid intermediate which was involved in the catalytic cycle. Key words plus:CROSS-COUPLING REACTIONS; RING-EXPANSION; MIGRATORY INSERTION; DIAZO-COMPOUNDS; BETA-LACTAMS; H ACTIVATION; DIAZOCARBONYL COMPOUNDS; METAL CARBENE; TOSYLHYDRAZONES; ACIDS 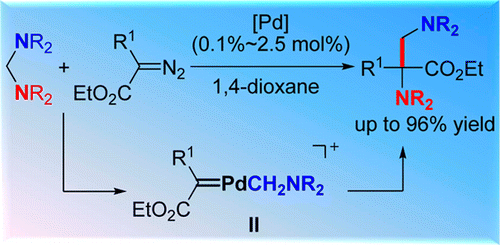
Published in JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, 137 (39):12490-12493; 10.1021/jacs.5b08476 OCT 7 2015 |

