Abstract: The mechanism-oriented reaction design for the divergent synthesis of chiral molecules from simple starting materials is highly desirable. In this work, aromatic amide-derived nonbiarylatropisomer/silver (silver/Xing-Phos) complex was used to catalyze the Michael addition of glycine aldimino esters to chalcones and successfully applied to the subsequent cyclocondensation to afford substituted cis-(1)-pyrroline derivatives with up to 98%ee. Besides the inherent performance of the chiral Ag/Xing-Phos catalyst system, it was found that the workup of such reactions played an important role for the stereoselective construction of stereodivergent (1)-pyrrolines, in which an epimerization of the cis-(1)-pyrrolines to the trans-isomers during was revealed. KeyWords Plus: ALPHA-AMINO-ACIDS; ENANTIOSELECTIVE 1,3-DIPOLAR CYCLOADDITIONS; PHASE-TRANSFER CATALYSTS; NON-BIARYL ATROPISOMERS; GLYCINE IMINO ESTER; CONJUGATE ADDITION; STEREODIVERGENT SYNTHESIS; AZOMETHINE YLIDES; SCHIFF-BASE; CALCIUM COMPLEXES Published in CHEMISTRY-A EUROPEAN JOURNAL, 22 (30):10399-10404; 10.1002/chem.201601945 JUL 18 2016 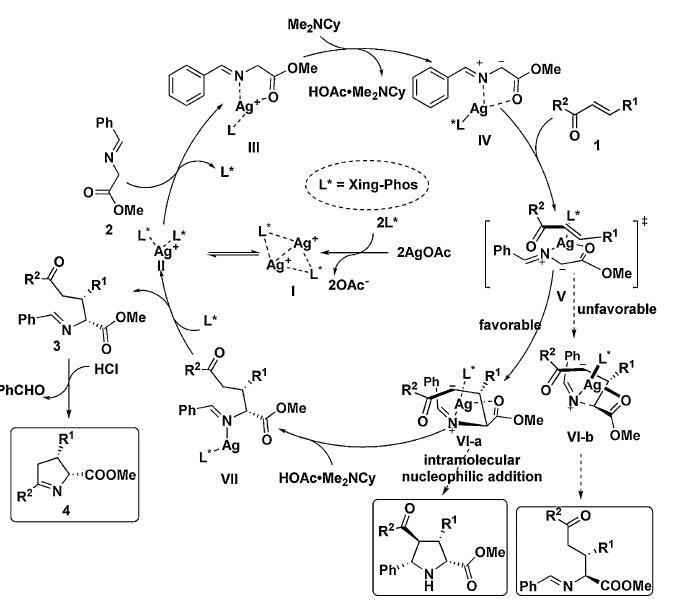
|

