The researchers from nuclear chemistry, Institute of Modern Physics (IMP), Chinese Academy of Sciences cooperated with University of Florida etc. realized the oxygen-content controllable graphene oxide (GO) preparation through high energy electron beam irradiation. The prepared GO was further used in lead sorption from water solutions.
Graphene oxide (GO) has attracted a significant interest in the field of sorption recent years because of its high specific surface area, excellent chemical stability and mechanical strength. GO has been used as an effective sorbent for heavy metal ions and organic contaminants removal from water solutions. All of the results suggested that the oxygen containing functional groups (carboxyl, epoxy and hydroxyl groups) on the surface of GO played an important role in the containments elimination. Hence, if more oxygen containing functional groups can be introduced into the GO structure, its sorption capacity for hazardous materials will certainly increase. Moreover, GO with high oxygen content will warrant its good solubility and be used as a good candidate in other fields.
Defects with different extent can be generated in the graphite structure by high-energy electron beam irradiation. Oxygen containing functional groups are more inclined to graft on the defect position of the carbon lattice when preparing GO by strong acid oxidation of graphite. Considering this characterization, graphite was irradiated under the electron beam generated from the high energy electron accelerator fabricated by Institute of Modern Physics, Chinese Academy of Sciences. The electron beam intensity was 6.25 mA and acceleration voltage was 1.5MeV. By changing the irradiation time, graphite with different irradiation dosages(0kGy, 6.4kGy and 19.2kGy)were obtained and then used for GO preparation. TEM, AFM, Raman spectrum and XPS analysis of the fabricated GO sheets showed that the oxygen containing groups, especially −OH and –COOH groups, grafted on the GO surfaces increased with increasing irradiation dosages. Therefore, oxygen-content controllable synthesis of GO can be achieved by adjusting the irradiation dosages. The obtained GO with different irradiation dosages were used for Pb(II) sorption. The effects of time, initial Pb(II) concentration, pH and ion strength on Pb(II) removal were investigated and lead sorption capacities were found to be increased with increasing oxygen content. The highest adsorption capacity was determined to be 636.4 mg/g at irradiation dosages of 19.2kGy which is much higher than most carbon based adsorbent reported in the literature.
This project is supported by the National Natural Science Foundation of China and China Scholarship Council. The work has been published in ACS Applied Materials and Interfaces 2016, 8, 25289−25296。
The article can be linked as follows:
http://pubs.acs.org/doi/suppl/10.1021/acsami.6b08059
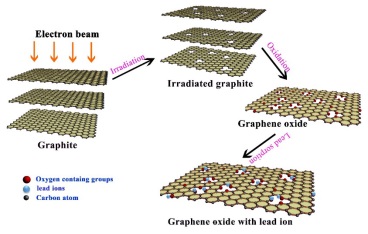
Fig.1 Preparation of graphene oxide (GO) and Pb(Ⅱ) sorption (Image by IMP)
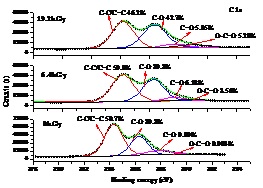
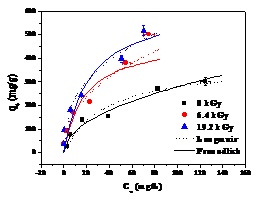
Fig.2 The C1s XPS spectra of prepared GO sheets (a) and Pb(Ⅱ) sorption results by different GO sheets (b) (Image by IMP)

