Uranium dioxide nuclear fuel has been widely used in pressurized water reactors (PWR), because of its high melting point, isotropic expansion, excellent radiation behaviors and mechanical properties. However, it is easy to embrittlement and the thermal conductivity is quite low. Compared with traditional uranium oxides nuclear fuel, the uranium carbide has extremely high hardness and no phase transition occurs in wide temperature range. The thermal conductivity, density and uranium fraction of UC is 21.7 W/(m·K) (1237 K), 13.63 g/cm3 and 95.2% respectively, which are much higher than that of UO2. Uranium carbide ceramic fuel has been considered as a potential candidate nuclear fuel for the next-generation fast-neutron reactors, especially for the Accelerator-Driven Systems (ADS). Based on the accelerator driven recycling used nuclear fuel, uranium carbide can recrystallize with plutonium (Pu) and minor actinides (MAs). Therefore uranium carbide is chosen as the fuel format in ADS.
Recently, homogeneous ceramic nuclear microspheres and UC powder have been successfully prepared in Qin Zhi’s group, using an improved microwave-assisted rapid internal gelation process and Pechini-type in situ polymerizable complex method respectively.
Firstly, uniform sized ceramic UC microspheres with a diameter of 675 ± 10 μm were successfully fabricated by an improved microwave-assisted rapid internal gelation process combined with carbothermic reduction (Figure 1). First of all, the nanoparticle carbon was dispersed into the HMUR stock solution, and the C-UO3·2 H2O gelled microspheres were prepared using an improved microwave-assisted internal gelation process without cooling the initial stock solutions. Next, the gelled microspheres were subjected to a carbothermic reduction process to obtain ceramic UC microspheres. This method will be potentially used for the fabrication of MAs and Pu-containing ceramic nuclear fuel microspheres at large scale in glove-box for the ADS project in future.
Secondly, Pechini-type in situ polymerizable complex method was optimized to synthesize UC powder (Figure 2). Citric acid was used as a chelating agent to UO22+ and mannitol was chosen as a polymerization agent. Compared with the conventional carbothermal reduction, a homogeneous and intimate mixing of the uranium and carbon sources in a molecular scale was achieved, leading to shorter diffusion distance and hence lower reaction temperature in subsequent carbothermal reduction.
This work was financially supported by the CAS Strategic Priority Research Program and the Chinese National Nature Science Fund (Advanced transmutation nuclear fuels: design, fabrication and properties). This work has been published in the international top journal Ceramics International and Journal of the American Ceramic Society. The first author is Dr. Tian Wei and Guo Hangxu respectively.
The articles can be linked as follows:
https://www.sciencedirect.com/science/article/pii/S0272884218317152
https://onlinelibrary.wiley.com/doi/abs/10.1111/jace.15438
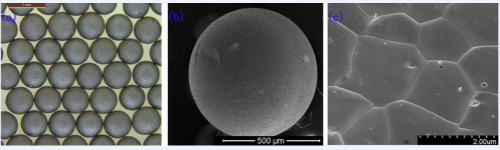
Figure 1: Ceramic UC spheres fabricated by the improved microwave-assisted rapid internal gelation process. a: optical image of the ceramic UC microspheres; b: SEM image of the ceramic UC microspheres; c: micro-morphology of the ceramic UC microspheres.
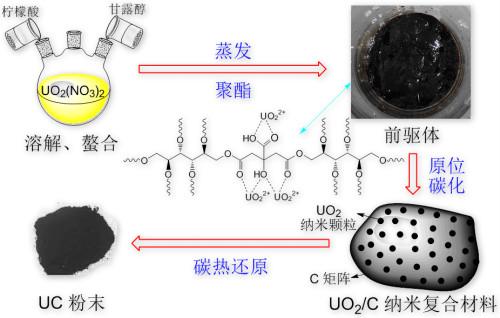
Figure 2:Flowchart of preparation UC powder using Pechini-type in situ polymerizable complex method. Firstly, UO22+-CA complexes were obtained by the chelation of citric acid (CA) to UO22+; secondly, porous precursor material was obtained through the evaporation of solvent and the polyesterification between UO22+-CA complexes and mannitol; subsequently, UO2/C nanocomposites were gained by in-situ charring; finally, UC powder was obtained via carbothermal reduction.

