Reprocessing of spent nuclear fuel has became the key issues for the environmentally friendly and sustainable development of nuclear energy. With the help of the external neutron source, the rest spent nuclear fuel after removing the part fission products will be refabricated to new nuclear fuel and burned. For those volatiles and semi-volatile fission products would be easily removed by using a dry-processing technique processes. For lanthanides, which accounts for about 1/3 of the fission products, must be separated from minor actinides before next transmutation step because the high neutron capture cross sections of some lanthanides, named as neutron poisons, inhibit efficient transmutation. However, mutual separation of MA and Ln is very different due to their similar chemical properties. The researchers describe herein an environmentally benign strategy for removing of fission products from spent nuclear fuel by the selective dissolution using carboxyl-functionalized ionic liquid [Hbet][Tf2N]. Water-statured [Hbet][Tf2N] can dissolve lanthanides oxide from simulated spent nuclear fuel with a dissolution ratio of 100% at 40℃. However, the dissolution of uranium is almost neglect (<1%) under the same conditions. This process of separation displays an outstanding performance to separate some key fission products oxide such as neutron poisons Ln2O3 efficiently and allows the recovery of actinides AnO2 as a group in solid form. Less volume of high level radioactive liquid waste (HLW) was generated during this process and there was no need for dissolving spent nuclear fuel since only some fission products was dissolved selectively and separated. The dissolved Nd and U were recovered efficiently from the loaded ionic liquid phase by back-extraction with 1M HCl solution. The ionic liquid phase can be reused again for the dissolution of Nd2O3 after washing off the residual acid with water. The high Ln2O3 dissolution capability is elucidated by thermodynamical analysis on the microprocess of the solubilizing and the U/x value related to the lattice energy U for MxOy lattice disruption. Figure 1 shows the novel approach to reprocessing spent nuclear fuel based on the selective dissolution and separation using [Hbet][Tf2N and the U/x value related to the lattice energy U for MxOy. This work represents the first case for efficient fission products removal by selective dissolution, avoiding the complete dissolution of spent nuclear fuel, the producing of the large high-level radioactive waste and reducing environmental hazards. Moreover, this approach would enhance proliferation resistance significantly because sole Pu would not be separated. The present method provides a facile, environmental friendly and high efficiency fission products separation strategy from spent nuclear fuel and introduces a new separation approach to the spent nuclear fuel reprocessing. This work has been published in Inorganic Chemistry. The work was financially supported by the CAS Strategic Priority Research Program and Young Scholar of CAS “Light of West China” Program. The articles can be linked as follows: https://pubs.acs.org/doi/10.1021/acs.inorgchem.8b02783 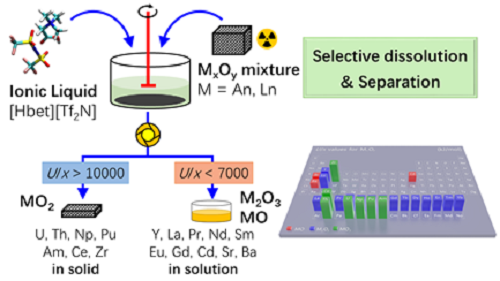
Fig. 1 the novel approach to reprocessing spent nuclear fuel based on the selective dissolution and separation using [Hbet][Tf2N] |

