Optically active aminocyclopropane and its derivatives are ubiquitous subunits of many biologically active natural products, pharmaceuticals, and agrochemicals. Therefore, the asymmetric synthesis of aminocyclopropane has attracted considerable attention. Traditional catalytic asymmetric methods rely on [2+1] cycloaddtion of enamine dertivatives and hydro(carbo) amination of cyclopropenes. The reactive sites of these substrates need to be pre-functionalized, which often requires tedious steps and extra reagents. Thus, it is highly demanded to develop atom and step economic catalytic asymmetric method.The XU Senmiao’s group (https://www.x-mol.com/groups/senmiaoxu) from the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences has been focusing on transition-metal-catalyzed regio- and stereoselective C-H borylation for years. They have developed a novel class of chiral bidentate boryl ligands (CBL). The CBL/Ir catalytic system has shown remarkable success in asymmetric C-H borylation reactions of diarylmehylamines, cyclopropanecarboxamides, cyclobutanes, azacycles, acyclic amides, pyrazole derivatives, diarylphosphinates (ACS Catal. 2021, 11, 13445) and ferrocenes (ACS Catal. 2022, 12, 1830). 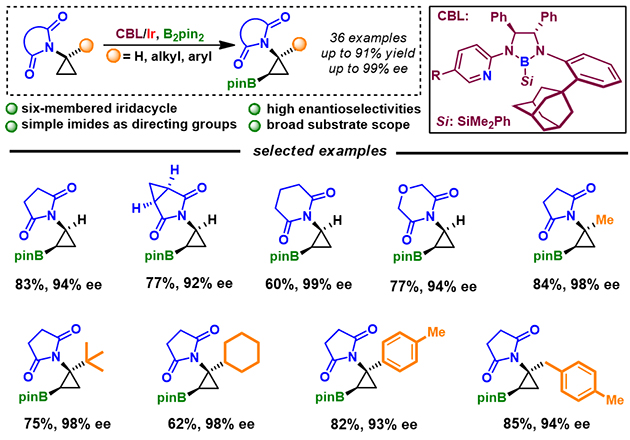
Figure 1. CBL/Ir-Catalyzed Asymmetric C(sp3)-H Borylation of Aminocyclopropanes Recently, the group has disclosed a new protocol for the simple imide-directed Ir-catalyzed C(sp3)-H borylation of aminocyclopropanes using adamantyl substituted CBL for the first time (Figure 1), overcoming challenges of the lack of suitable chiral catalysts and feasible directing groups, and competitive side reactions. A variety of functional groups could be well-tolerated, affording corresponding borylated products with up to 99% ee. They have also demonstrated that synthetic utility on a preparative scale C-H borylation for diverse transformations, including the synthesis of a nematicide candidate. The work is supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangsu Province, Lanzhou Institute of Chemical Physics, State Key Laboratory for Oxo Synthesis and Selective Oxidation, and Hangzhou Normal University.
Contact:
Xu Senmiao
Email: senmiaoxu@licp.cas.cn
Lanzhou Institute of Chemical Physics |

